16037-91-5
DEFINITION
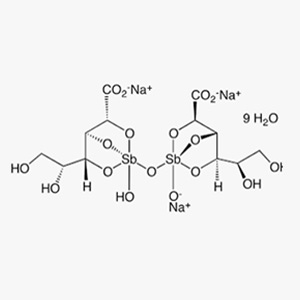
Sodium Stibogluconate is mainly the disodium salt of μ-oxy-bis[gluconato(3-)-c2,c3,c4hydroxoantimony]. It contains not less than 30.0% and not more than 34.0% of antimony(V), calculated with reference to the dried and methanol-free substance.
PRODUCTION
The method of manufacture is such as to ensure consistently controlled reaction stoichiometry in order to yield sodium stibogluconate that is satisfactory with regard to intrinsic toxicity.
CHARACTERISTICS
A colourless, mostly amorphous powder.
Very soluble in water; practically insoluble in ethanol (96%) and in ether.
IDENTIFICATION
A. A solution is dextrorotatory.
B. Pass hydrogen sulphide into a 5% w/v solution for several minutes. An orange precipitate is produced.
C. When heated, it chars without melting leaving a residue which yields the reactions characteristic of antimony compounds and the reactions characteristic of sodium salts.