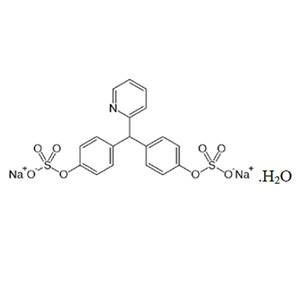
Sodium picosulfate also known as sodium picosulphate is a contact stimulant laxative used as a treatment for constipation or to prepare the large bowel before colonoscopy or surgery.
Ph Eur
C18H13NNa2O8S2,H2O --- 499.4 --- 10040-45-6
Action and use: Stimulant laxative.
DEFINITION
Sodium picosulfate contains not less than 98.5 per cent and not more than the equivalent of 100.5 per cent of 4,4¢-(pyridin-2-ylmethylene)bisphenyl bis(sodium sulphate), calculated with reference to the anhydrous substance.
CHARACTERS
A white or almost white, crystalline powder, freely soluble in water, slightly soluble in alcohol.
IDENTIFICATION
First identificationiA, E.
Second identificationiB, C, D, E.
A. Examine by infrared absorption spectrophotometry (2.2.24), comparing with the spectrum obtained with sodium picosulfate CRS. Examine the substances prepared as discs.
B. Examine the chromatograms obtained in the test for related substances in ultraviolet light at 254 nm. The principal spot in the chromatogram obtained with test solution (b) is similar in position and size to the principal spot in the chromatogram obtained with reference solution (a).
C. To 5 ml of solution S (see Tests) add 1 ml of dilute hydrochloric acid and heat to boiling. Add 1 ml of barium chloride solution. A white precipitate is formed.
D. To about 10 mg add 3 ml of sulphuric acid and 0.1 ml of potassium dichromate solution
R1. A violet colour develops.
E. The solution S gives reaction (a) of sodium (2.3.1).
TESTS
Solution S: Dissolve 2.5 g in distilled water R and dilute to 50 ml with the same solvent.
Appearance of solution: Solution S is clear and not more intensely coloured than reference.
Acidity or alkalinity: To 10 ml of solution S add 0.05 ml of phph solution. The solution is colourless. Not more than 0.25 ml of 0.01 M sodium hydroxide is required to change the colour of the indicator to pink.
Related substances: To pass the test.
Chlorides: Dilute 5 ml of solution S to 15 ml with water. The solution complies with the limit test for chlorides (200 ppm).
Sulphates: Dilute 7.5 ml of solution S to 15 ml with distilled water R. The solution complies with the limit test for sulphates (400 ppm).
Heavy metals: 12 ml of solution S complies with limit test A for heavy metals (20 ppm).
Water: 3.0 per cent to 5.0 per cent, determined on 0.500 g by the semi-micro determination of water.
ASSAY
Dissolve 0.400 g in 80 ml of methanol. Titrate with 0.1 M perchloric acid, determining the end-point potentiometrically.
1 ml of 0.1 M perchloric acid is equivalent to 48.14 mg of C18H13NNa2O8S2.
IMPURITIES
A. R = SO3Na: 4-[(pyridin-2-yl)(4-hydroxyphenyl)methyl]phenyl sodium sulphate,
B. R = H: 4,4¢-[(pyridin-2-yl)methylene]bisphenol.
C18H13NNa2O8S2-H2O --- 499.42
C18H13NNa2O8S2 --- 481.41
4,4′-(2-Pyridylmethylene)diphenyl bis(hydrogen sulfate) disodium salt, monohydrate;
Disodium 4,4′-(pyridin-2-ylmethanediyl)dibenzenesulfate
4,4′-(Pyridin-2-ylmethylene)bisphenyl bis(sodium sulfate), monohydrate --- [10040-45-6]; UNII: VW106606Y8.
DEFINITION
Sodium Picosulfate contains NLT 98.5% and NMT 100.5% of sodium picosulfate (C18H13NNa2O8S2), calculated on the anhydrous basis.
IDENTIFICATION
A. Infrared Absorption
B. Identification Tests—General, Sodium: Meets the requirements for the pyroantimonate precipitate test
ASSAY
Sample solution: Dissolve 400 mg of Sodium Picosulfate in 80 mL of methanol.
Analysis: Titrate with 0.1 N perchloric acid determining the endpoint potentiometrically. Perform a blank determination, and make any necessary correction. Each mL of 0.1 N perchloric acid is equivalent to 48.14 mg of sodium picosulfate (C18H13NNa2O8S2).
Acceptance criteria: 98.5%–100.5% on the anhydrous basis
IMPURITIES
Chloride and Sulfate, Chloride: A 1.0-g portion shows no more chloride than corresponds to 0.30 mL of 0.020 N hydrochloric acid: NMT 0.02%.
Chloride and Sulfate, Sulfate: A 500-mg portion shows no more sulfate than corresponds to 0.20 mL of 0.020 N sulfuric acid: NMT 0.04%. >
Organic Impurities: To pass the tests
Acidity and Alkalinity: To 10 mL of the portion of Sample solution retained from the test for Color of Solution add a drop of phph. The solution is colorless. NMT 0.25 mL of 0.01 N sodium hydroxide is required to change the color of the indicator to pink.
Water: 3.0%–5.0%.