Ph Eur
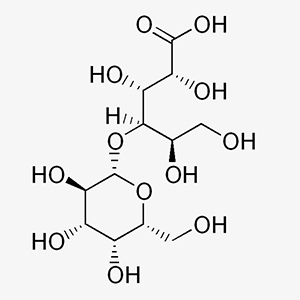
C12H22O12 (acid form) -- 358.3 -- 96-82-2
C12H20O11 (d-lactone) -- 340.3 -- 5965-65-1
DEFINITION
Mixture in variable proportions of 4-O-b-D-galactopyranosyl-D-gluconic acid and 4-O-b-Dgalactopyranosyl-D-glucono-1,5-lactone.
Content: 98.0 per cent to 102.0 per cent (anhydrous substance).
CHARACTERS
Appearance: White or almost white powder.
Solubility: Freely soluble in water, slightly soluble in glacial acetic acid, in anhydrous ethanol and in methanol.
mp: About 125C with decomposition.
IDENTIFICATION
A. Infrared absorption spectrophotometry.
Comparisonılactobionic acid CRS.
If the spectra obtained show differences, dissolve the substance to be examined and the reference substance separately in water, dry at 105C and record new spectra using the residues.
B. Thin-layer chromatography.
Test solution: Dissolve 10 mg of the substance to be examined in water and dilute to 1 ml with the same solvent.
Reference solution: Dissolve 10 mg of lactobionic acid CRS in water and dilute to 1 ml with the same solvent.
Plate TLC silica gel plate.
Mobile phase concentrated ammonia, ethyl acetate, water, methanol.
Application: 5 μl.
Development: Over 3/4 of the plate.
Detection: Spray 3 times with ammonium molybdate solution and heat in an oven at 110C for 15 min.
Results: The principal spot in the chromatogram obtained with the test solution is similar in position and colour to the principal spot in the chromatogram obtained with the reference solution.
TESTS
Appearance of solution: The solution is clear and not more intensely coloured than reference.
Specific optical rotation: + 23.0 to + 29.0 (anhydrous substance).
Dissolve 1.0 g in 80 ml of water and dilute to 100.0 ml with the same solvent. Allow to stand for 24 h.
Reducing sugars: Maximum 0.2 per cent, calculated as glucose.
Dissolve 5.0 g in 25 ml of water  with the aid of gentle heat. Cool and add 20 ml of cupricitric solution and a few glass beads. Heat so that boiling begins after 4 min and maintain boiling for 3 min. Cool rapidly and add 100 ml of a 2.4 per cent V/V solution of glacial acetic acid and 20.0 ml of 0.025 M iodine. With continuous shaking, add 25 ml of a mixture of 6 volumes of hydrochloric acid and 94 volumes of water and, when the precipitate has dissolved, titrate the excess of iodine with 0.05 M sodium thiosulphate using 1 ml of starch solution, added towards the end of the titration, as indicator. Not less than 12.8 ml of 0.05 M sodium thiosulphate is required.
Heavy metals: Maximum 20 ppm.
Water: Maximum 5.0 per cent, determined on 0.50 g.
Total ash: Maximum 0.2 per cent.